Why Does Silicon Dioxide Have High Melting Point
Because the silicon and oxygen atoms are held. Why does silicon dioxide have a higher melting point than sulphur.
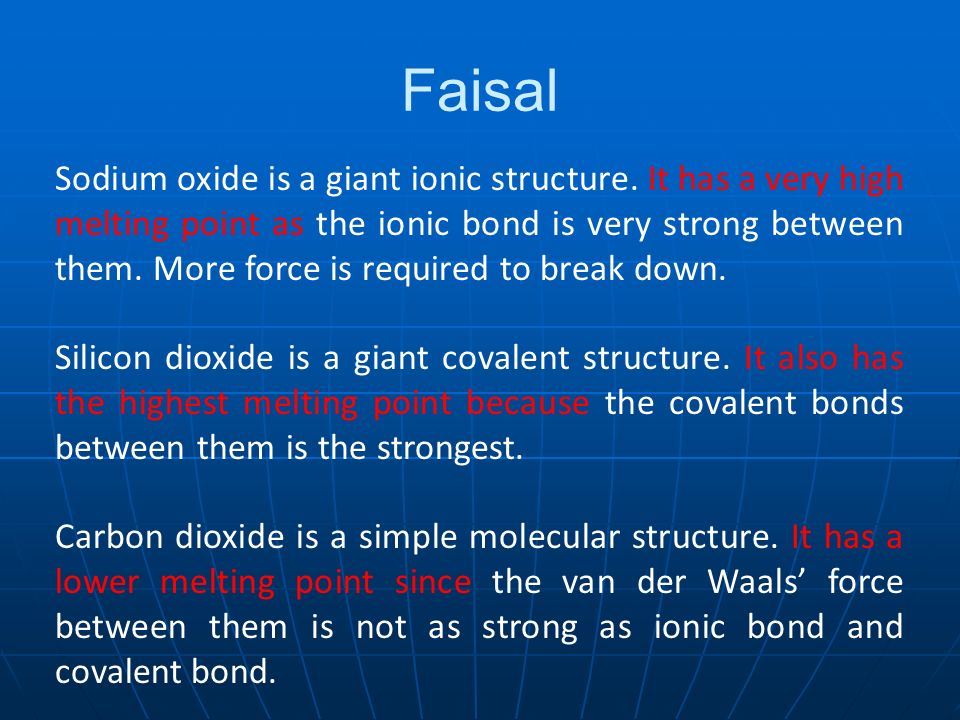
Based On Their Structures Explain Why Sodium Oxide Silicon Dioxide And Carbon Dioxide Have Different Melting Points Here Are 6 Real Student Answers Ppt Download
Silicon dioxide SiO2 has a macromolecular structure.

. This means that it forms thousands of covalent bonds between its silicon and oxygen subunits. Silicon has a very high melting point due to its giant covalent structure. Why does silicon dioxide have a high melting point.
Argon exists as individual atoms with weak van der Waals. Why does silicon dioxide have a high melting and boiling point. SiO2 has a high melting point.
This means that it forms thousands of covalent bonds between its. Silicon dioxide has a higher melting point than sodium chloride. Silicon IV oxide or SiO2 has a high melting point because it is a solid.
Silicon dioxide exists as a giant covalent structure where each Si atom is connected to four oxygen atoms and. A lot of energy is needed to break the strong covalent bonds throughout the structure. Why does silicon dioxide have a high melting point.
Thus the melting temperature of silicon is higher as it exists as a giant covalent structure so strong covalent bonds have to be broken in order to melt it whereas phosphorus. In terms of crystal structure and bonding explain why the melting point of silicon dioxide is high. Why does silicon dioxide have a high melting point.
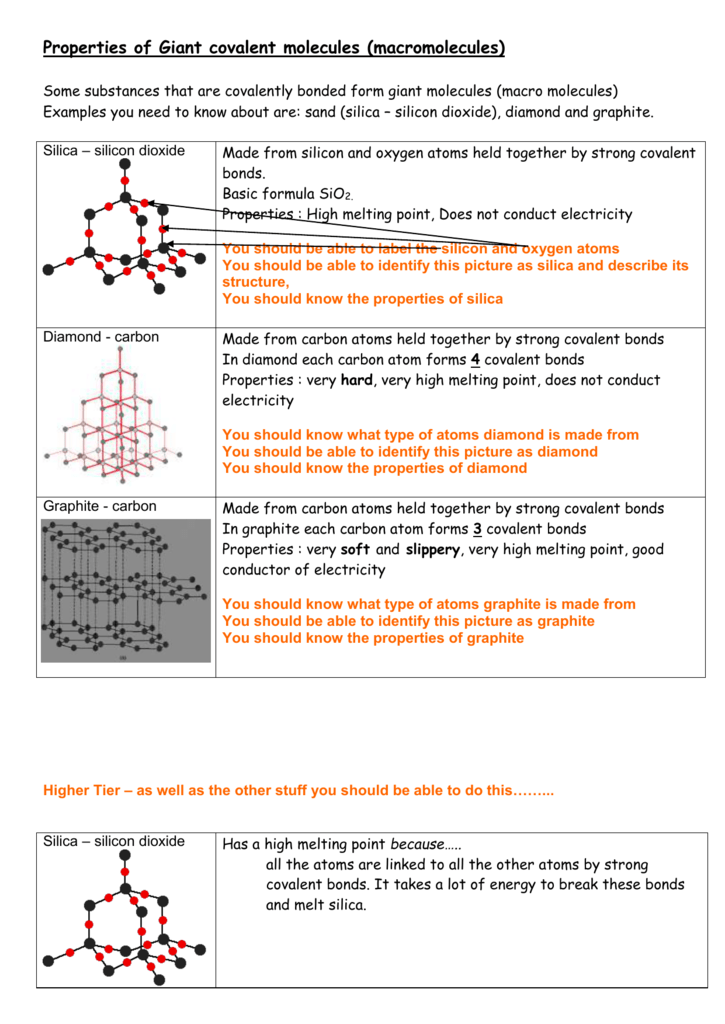
High melting and boiling point. Many strong covalent bonds. A giant covalent molecule.
Sulfur Trioxide has a simple molecular structure. Silicon dioxide SiO2 has a macromolecular structure. Silicon has a very high melting point due to its giant covalent structure.
In comparison carbon dioxide is a gas at room temperature. Silicon Dioxide has a macromoleculargiant covalent structure which means it has covalent bonds between all atoms in its structure. A lot of energy is needed to break the strong covalent bonds throughout the structure.
Macromolecular Strong covalent bonds between atoms Predict whether the melting point of. Since covalent bonds require more. Why does silicon dioxide have such a high melting point.
Has a high melting point - varying depending on what the particular structure is remember that the structure given is only one of. SiO_2 is a homologue of CO_2 a molecular room temperature gas. The physical properties of silicon dioxide.
Lots of energy is needed to overcome those strong covalent bonds. Silicon dioxide has an exceptionally high melting point which you should look up and report. The giant structures the metal oxides and silicon dioxide will have high melting and boiling points because a lot of energy is needed to break the strong bonds ionic or.
Because the silicon and oxygen atoms are held by strong intermolecular forces. This is due to the strength of Si-O-Si binds in the lattice.

Why Is Carbon Dioxide A Gas While Silicon Dioxide A Solid Quora

Quick Answer Why Does Silicon Carbide Have A High Melting Point Seniorcare2share
![]()
Macromolecules Covalent Network Solids Last Part Of Topic Ppt Download
0 Response to "Why Does Silicon Dioxide Have High Melting Point"
Post a Comment